Abstract
Introduction Hematopoietic stem cell transplantation (HSCT) is currently the only curative treatment for patients with sickle cell disease (SCD). Whereas several studies have shown that the markers of anemia and hemolysis normalize after HSCT, little is known about the effects of HSCT on the parameters of cerebral perfusion and oxygen metabolism in adult patients with SCD. In patients with SCD, cerebral blood flow (CBF) is increased to compensate for anemia in order to maintain adequate oxygen delivery. However, despite this, oxygen extraction and consumption have been found to be lower compared to controls. So far only a single study in four patients with SCD reported that the CBF and oxygen extraction fraction (OEF) normalized after HSCT. In this work, we analyzed the effect of HSCT on CBF, cerebrovascular reactivity (CVR), OEF and cerebral metabolic rate of oxygen (CMRO2) in addition to the hematological parameters in adult patients with SCD before and after HSCT.
Methods We included 10 patients (7 HbSS, 2 HbSβ0 and 1 HbSβ+; mean age: 28.3 years (19-45), 3 female) who were studied 15-246 days before HSCT and 6-34 months after non-myeloablative (alemtuzumab/3 Gy total body irradiation) matched sibling donor HSCT following pre-conditioning with azathioprine and hydroxyurea. Gray matter (GM) and whole brain (WB) CBF were measured using time-encoded pseudo-continuous arterial spin labeling (te-ASL). Venous blood T2 in the superior sagittal sinus was measured using T2-Relaxation-Under-Spin-Tagging. Te-ASL was performed before and after a vasodilatory stimulus using acetazolamide (ACZ). OEF was calculated from arterial saturation measured by pulse oximetry and venous saturation calculated from blood T2 using a sickle cell-specific calibration model before HSCT and a healthy control model after HSCT. CMRO2 was calculated using the following equation:
CMRO2 = WB CBF × OEF × oxygen content
where oxygen content was calculated from hemoglobin and arterial saturation.
CVR reflects the capacity of the blood vessels to dilate in response to a vasoactive stimulus and was calculated using the following equation:
CVR = (GM CBFpost-ACZ - GM CBFpre-ACZ) / GM CBFpre-ACZ × 100%.
where CBFpre-ACZ and CBFpost-ACZ are the CBF measured before and after ACZ respectively.
A Wilcoxon signed-rank test was used to test the significant differences in hemoglobin, reticulocytes, LDH, total bilirubin, CBF, CVR, OEF and CMRO2 before and after HSCT.
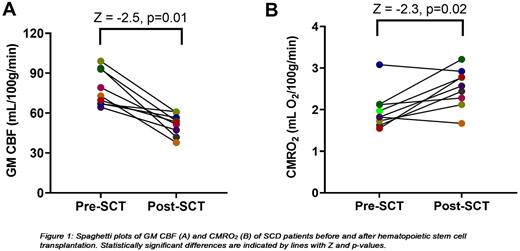
Results CBF, CVR and CMRO2 of one patient were excluded because of severe motion during the te-ASL scan. Hemoglobin levels were significantly increased (Z = -2.8; p<0.01) and markers of hemolysis (LDH (Z = -2.7; p<0.01), reticulocytes (Z = -2.8; p<0.01) and total bilirubin (Z = -2.8; p<0.01)) were significantly reduced after transplantation. GM CBF significantly decreased after HSCT (Fig. 1A) while GM CVR increased in all patients (Z = -2.7; p<0.01). OEF (Z = -2.2; p= 0.03) and CMRO2, parameters of oxygen metabolism, significantly increased after transplantation (Fig. 1B). One patient ( HbSβ+) who had been using hydroxyurea for years before transplantation showed a remarkable high OEF before transplantation (45.6%) which in contrast to other patients reduced after transplantation (36.2%). Since both CBF and OEF decreased after transplantation in this patient, CMRO2 slightly decreased after transplantation (3.1 vs 2.9 mL O2/100g/min; dark blue dot in Fig. 1B). Another patient also showed a slightly lower CMRO2 after transplantation (1.82 vs 1.67 mL O2/100g/min) which was caused by a strong reduction in CBF which was halved after transplantation (orange dot in Fig. 1A-B).
Conclusion Cerebral blood flow normalized after HSCT in adult SCD patients. In addition, we found increased GM CVR after HSCT suggesting that the vasodilatory capacity of the cerebral vessels might be increased compared to before transplantation. After transplantation, cerebral oxygen metabolism, reflected by OEF and CMRO2, was also restored to values reported in healthy controls, indicating improved cerebral oxygen utilization in these patients. Looking ahead, follow-up scans need to be performed to determine whether this improved cerebral oxygen metabolism will prevent future cerebral (silent) infarctions.
Disclosures
Nur:Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Biemond:Bluebird Bio: Membership on an entity's Board of Directors or advisory committees; Chiesi: Membership on an entity's Board of Directors or advisory committees; CSL Behring: Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; GBT: Research Funding; Bluebird Bio: Membership on an entity's Board of Directors or advisory committees; CSL Behring: Membership on an entity's Board of Directors or advisory committees; Chiesi: Membership on an entity's Board of Directors or advisory committees; Modus Therapeutics: Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; GBT: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanquin: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.